
Understanding Technology Readiness Levels Framework for Medical Countermeasure Development
Technology Readiness Levels (TRLs) provide a standardized framework to assess the **maturity** of medical countermeasure products, ensuring they are ready for deployment in critical scenarios. Originating from NASA, TRLs are now integral in guiding the development and regulatory pathways of diagnostics and other medical devices.
Key Takeaways
- TRLs offer a **common language** to describe technology maturity without requiring technical expertise.
- There are nine distinct levels, each representing a stage in the **development** and validation process.
- TRLs 6-7 are critical for demonstrating prototypes in operational environments.
- Understanding TRLs aids in making informed **funding** and investment decisions.
- While TRLs provide structure, they may require **adaptations** for specific medical applications.
Understanding Technology Readiness Levels (TRLs) in Medical Countermeasures
Technology Readiness Levels (TRLs) are standardized numerical indicators that measure the **maturity** of a technology from inception to full deployment. Initially developed by NASA in the 1970s for space technology, TRLs have been adopted by major organizations like the U.S. Department of Health and Human Services and the Department of Defense to streamline the development process of medical devices and diagnostics.
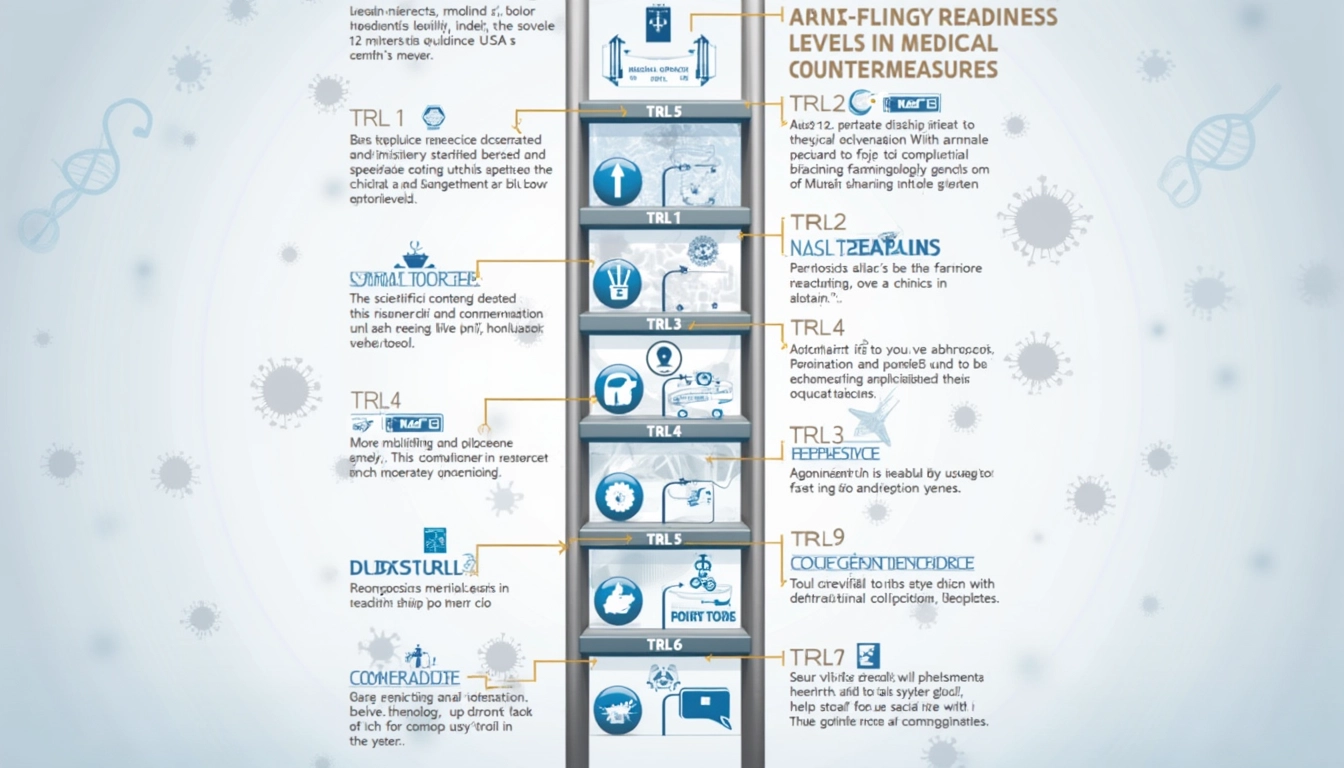
The Nine Levels of Technology Readiness
TRL 1: Basic Principles Observed and Reported
At TRL 1, scientific research begins its translation into potential applications. This foundational stage involves the active monitoring of the scientific knowledge base, such as the basic identification of opportunities and the review of human disease pathology.
TRL 2-3: Concept Formulation and Experimental Validation
TRL 2 marks the inception of an invention where practical applications are still speculative. By TRL 3, active research and development are underway, including laboratory studies, hypothesis formulation, and computer simulations. Examples include threat agent identification and exploratory studies.
TRL 4-5: Component Integration and Validation
During TRL 4, basic technological components are integrated in a laboratory setting. Moving to TRL 5, the fidelity increases as the technology scales up to pilot quantities, ensuring compliance with Good Manufacturing Practices (GMP). Activities include chemical profile optimization and stability testing.
Critical Stages: TRL 6-7 for Medical Countermeasures
TRL 6 involves the demonstration of a system or subsystem prototype, including GMP-compliant pilot lot production and Phase 1 clinical trials. TRL 7 advances to prototype demonstration in an operational environment, encompassing Phase II clinical trials and chronic toxicity testing.
Final Stages and Commercial Deployment: TRL 8-9
At TRL 8, the actual system is completed and qualified through full-scale testing and regulatory compliance. TRL 9 signifies that the system has been proven in mission operations, with continuous production and rigorous quality control measures in place.
Importance and Limitations of TRLs in Medical Countermeasures
TRLs are essential for **risk management**, ensuring that technology reaches adequate maturity before system incorporation. They also guide funding decisions based on technology maturity and ensure regulatory compliance with GMP and GLP requirements. However, TRLs have limitations, such as generic definitions and a linear pipeline representation, necessitating sector-specific adaptations for medical countermeasures.
For instance, advancements in real-time gene activity monitoring can be better understood through the lens of TRLs, as demonstrated by innovations in gene activity monitoring technologies. Additionally, the integration of AI in medical devices highlights the evolving nature of TRLs in accommodating new technological paradigms.


